HealthBuilderS®
Professional Coaching
Functional Neurology Every Doctor Should Know!
Cortical release signs: the modified Galant reflex using applied kinesiology as functional neurology
Abstract
OBJECTIVE: To assess the involvement of the primitive, cortical or frontal release signs—specifically the Galant Reflex (GR)—on various manual muscle tests.
METHOD: Functionally evaluate the GR relative to various endogenous stimuli using manual muscle testing as functional neurology.
RESULTS: A Medline search to answer the question, “What does it mean to display a positive Galant reflex?” yielded several perspectives related to cortical release display but none that address their functional application in a clinical setting. Manual muscle testing (MMT) techniques can expose a number of primitive reflex abnormalities not otherwise obvious to the clinician. The prevalence of cortical release signs (CRSs) in static postural and dynamic gait conditions allows for their use in the clinical management of various patient populations, including diagnostic assessment, treatment monitoring and prognosis.
CONCLUSION: The GR is an integral part of the normal human neurological fabric at any age. Its pathological display is any functional dysfunction which devolves from that which is unencumbered. This evaluation technique complements the standardized neurological examination and may allow the use of MMT as an adjunct to the prediction of motor disability in patients of all ages. A number of functional issues complicate the proper interpretation of CRSs in clinical populations, not the least of which is the uncertainty over what constitutes an “abnormal” response.
INTRODUCTION
Primitive (frontal) reflexes make up some of the earliest, simplest, and most frequently used examination measures to assess the neonate. They are a group of involuntary motor responses normally found early in postnatal development and become intimately and inseparably integrated with the human neurologic fabric. Although their integration is constant, their influence may be “released” by cerebral, usually frontal, damage. (Schott & Rossor, 2003; Hyde et al, 2007) Their display becomes pathological when their control mechanisms are mistimed relative to their fundamentals.
developing associated structural and/or metabolic condition(s). The links between the clinical pictures, CRSs and brain pathology are made clearer with this understanding.Although a comprehensive neurologic examination is always indicated, the combined examination of primitive reflexes and postural reactions should be considered by the neurologist as a simple but predictive screening test for the early identification of functional neurological issues. A functional neurological examination can elicit many developmental disorders not detected by metabolic screening programs. These neurological screening tests are quick and easy to perform on anyone from neonate to elder adult.
The Muscle-Organ Relationship
Because of its relationship to the homologous intermediolateral cell column, the presence of a functionally dysfunctional GR [together with an aberrant deep tendon reflex (DTR), crossed cord reflex (CCR), or CRS—i.e., tonic neck (TNR), flexor withdrawal (FWR) reflexes] tends to indicate a very high probability that there is a concomitantly dysfunctional autonomic display that involves an associated muscle or muscles.
Further, because of the unique neurological effects of tapping and stroking the epidermis in various areas, there appears to be a concomitant effect on the cerebellum ipsilaterally and the thalamus contralaterally, respectively.
The Cortical Release Signs
By virtue of its homologously-related cord involvement, each of the CRSs has its effect on human movement patterns. They are undeniably involved and inextricably intertwined into the fabric of human movement. These reflexes, when not properly integrated into the maturing central nervous system, are associated with structural, functional, and autonomic problems. (Guzik et al, 2007; Bennett et al, 2002)
Joint reciprocity according to the predetermined human design schematic is crucial to functional autonomic display and vice versa. Appropriate and reciprocal joint motion afferentates the spinal cord and more rostral neuraxial centers according to their original intent, and that coordinates appropriate motor responses and autonomic modulation through efferent autonomic drive. This is illustrated in Table 1 on the previous page.
MATERIALS AND METHODS
The Galant Reflex (GR)
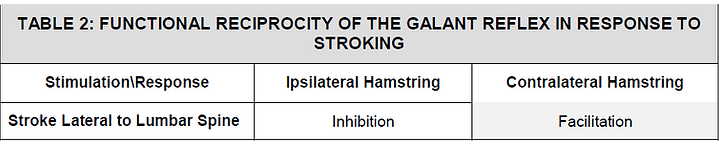
The GR is usually elicited by holding an infant who is prior to its seventh month of age in ventral suspension (face down) and stroking the skin along one side of the lower (below T6) spine. The normal response facilitates the ipsilateral rectus femorus causing the infant to flex at the hip and swing its hips towards the side that was stroked. This normal GR display is raw and unencumbered by other more experienced neurological development. It is said that the GR is pathological if its display persists past six months of age.
Reflexive facilitation of the rectus femorus causes a concomitant inhibition of the ipsilateral hamstring. Therefore, the GR can be used in a clinical setting to evaluate the dynamics of the ipsilateral rectus femorus/hamstring reciprocity. (See Table 2)
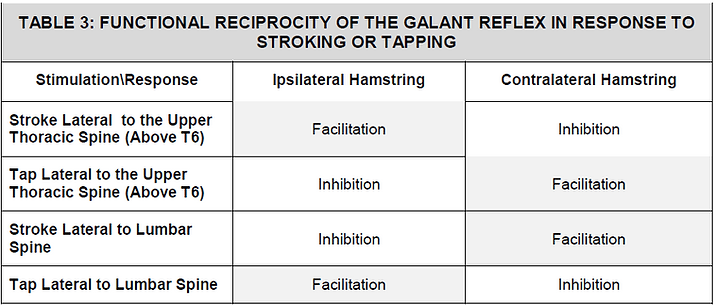
This paper expands on what has until recently been considered to be the normal GR display for more applicable use in the clinical scenario. This author has observed that stroking and tapping the lateral aspect of the lumbar spine with a sharp object or blunt instrument, respectively, has two different displays. The stroking stimulus causes the ipsilateral rectus femorus’ facilitation while the tapping causes its inhibition. Similarly, the crossed cord response causes the opposite display contralaterally. These same stroking and tapping techniques can also be used in the upper dorsal spine (above T6) with the opposite stimulation and display (Table 3).
When the outcome of the MMTs and their response to the GR tests conforms to their original design, one concludes that its display is nominal. Any other display must be considered pathological.
RESULTS
The GR evaluation consisted of a normal clinical setting. Each patient was tested relative to the GR testing procedures described above. A GR was considered positive when its functional display represented anything other than that which was anticipated by the nominal display. It was difficult to develop a control group because the soft neurological findings appeared ubiquitous. Only after treatment of the patient’s individual neurological findings did the dysfunction resolve—become nominal.
DISCUSSION
A number of issues complicate the interpretation of CRSs not the least of which are the myriad observations and interviews reported in the literature. A functional demonstration of the nominal cortical display in the non-clinical population resolves much of the argument. Using MMT as functional neurology appears to indicate that the understanding of CRSs should be reinterpreted, especially when their clinical significance is persistent and prominent in the examination. In some circumstances, CRSs may assist in diagnostic differentiation and illness staging.
Classical clinical-pathological correlations have suggested that CRSs in adults are one of the few bedside indices of prefrontal cortical dysfunction. (Hyde et al, 2007) If there is a functional display it must have an involved motor component that can be displayed with MMT.
Conditions of cortical release appear to have a greater neurological consequence relative to normative groups. (Egan et al, 2001b; Lawrie et al, 2001; Cuesta et al, 2002; Gourion et al, 2004) However, our data appeared to indicate that dysfunctional CRSs are more common and neurologically involved than previously demonstrated. Those patients who have movement disorders as demonstrated by an aberrant neuromuscular display have learned to utilize some aspect of their primitive reflexes to induce more reliable and predictable movements. This sensory-motor-sensory feedback may show that the persistent yet asymptomatic functional breakdown of the frontal system eventually leads to more profound neurological involvement.
Functional Reciprocity
Functional reciprocity is one of the hallmarks of normal human movement. All primary afferents initially cause excitation to all cord functions including the facilitation of all modulatory effects of the intermediolateral cell column allowing for the dilation of arterioles to muscle and capillaries to skin, and the stimulation of piloerector tissue and sweat glands. They also excite the primary inhibitory neurons that inhibit nociception. Further, their effect rises to midbrain and cerebellar centers. Finally, primary afferents affect all the rostral neuraxial confluence that has any bearing on the anterior horn (the final common pathway), whose effect is reciprocally displayed in each homologously related muscle linked to the initial input.
For the upper and lower extremities to be efficient, besides the shunt stability that makes it possible, the spinal cord signals must maintain reciprocal facilitation of the primary movers and their synergists and inhibition of the ipsilateral antagonists while simultaneously inhibiting and facilitating the muscles that do the opposite activity contralaterally.
Each pre-programmed movement reinforces the interconnectivity between receptors and effectors that maintain the spatial and temporal relationships among the component parts of the movement at the cord levels that share a similar or related structure, position, or function. The synchronicity of the cord with the cortex and midbrain allows for precise and balanced give-and-take motion. With long-term reciprocity, the amount of cortical influence lessens to the point that the component patterns homogenize until a single thought may be sufficient to initiate a whole train of motor events.
Once set, the movement becomes a feedback-regulated pattern that is in some way stored in the central nervous system fully initiated by a simple but specific input event. The sequence of motor actions can precede almost entirely the control of subcortical means. The functional learning process shifts the major portion of movement control from cortical to peripheral mechanisms.
However, movement patterns tend to degenerate secondary to gravitational influences and their concomitant structural asymmetries resulting in deafferentation of rostral centers and their consequential motor responses. The effect of that deafferentation can be elicited using manual muscle testing techniques. Proprioceptive responses can be instantly identified in motor skills inherent to the human genome, and their dysfunction can be quickly diagnosed using functional neurological examination procedures.
A functionally dysfunctional CRS will display itself in a muscle when that muscle is tested with manual muscle testing techniques as the result of a timing error—the adjustment of motor planning relative to the sequencing of the muscle function and joint motion such that maximum output power is achieved—somewhere between its receptor, primary afferent, cord involvement, rostral neuraxial centers, cortical outflow, or efferent response. These primitive displays are reflexive, submissive to the primary afferentation that ultimately dictates the integrity of the reflex’s display. Other testing procedures monitor the reflexive display but only functional neurological testing observes the efferent response relative to the afferent stimulus. This perspective is the key that distinguishes applied kinesiological procedures as a critical component of functional neurology.
Re-establishing functional central and peripheral reciprocity facilitates cortical integrity and synchrony. Overall, the extensive manual muscle tests helped elicit a physiological display necessary to understand the extent of the dysfunctional CRS’s involvement.
Efferent Autonomic Drive and Muscle Concomitants
Only 10% of the entire cortical outflow eventually reaches muscle via corticospinal pathways. Fully 90% of the cortical outflow effects autonomic concomitants via extrapyramidal pathways, and these extrapyramidal pathways modulate the pyramidal display. Of the 10% to muscle, the fibers of the anterior horn must maintain reciprocity lest the dorsal horn of the cord becomes deafferentated, and vice versa.
Each intact CRS has its characteristic display in the human system yet it remains unrecognized unless and until it is challenged. What does it mean to be a “cortical release sign” if that sign is not tested against the function of a muscle? How could a functionally dysfunctional CRS be evaluated other than relative to its functional expression? Each of these functional displays is important to the mature fabric of the entire human system. Their reciprocal effects influence the cord to allocate movement patterns that respond to the environmental and autonomic factors that support that response. Any influence that generates a dysfunctional motor display will also be accompanied by autonomic concomitants, both of which must ultimately be related to some deafferentation of the higher centers that would create that display, and that ultimately stems from the homologous peripheral receptors.
Further, and because the interdependence of the sensory and motor systems, any individual or combination of dysfunctional CRSs also has its/their negative effect on the integrity of the cortical outflow anywhere from the cortex and rostral mesencephalon to the intermediolateral aspects of the cord. Any deafferentation of the primary afferents—anywhere from the receptor to the cortex—creates a hemisphericity that ultimately displays itself in the performance of a muscle and the function of its related efferent autonomic drive. That outflow is through the extrapyramidal pathways to the cord to segmentally and homogously modulate the involved organs; i.e., organ, vascular, pH, oxygenation, and aerobic performance.
While there may have been an original structural concern, the primary functional lesion may arise from some other homologously-linked area indicating that the original complaint was simply a symptom of a greater picture of abnormal primary afferentation with concomitant dysfunctional efferent autonomic drive. However, when it came to treatment, we considered the global structural involvement and its concomitant autonomic display and not just the focal complaint.
A Receptor-Driven System
The function of the entire human nervous system is receptor-driven. (Godde et al, 2003; Weiss et al, 2004; Graziano et al, 2009; Llinás et al, 1999) The whole design is an incredibly dynamic and plastic milieu that is heavily influenced by primary receptor potentiation. Cortical integrity depends on the precision of these primary afferents, which mainly arise from tissues that are able to produce movement of body parts, maintain tension, or pump fluids within the body, influencing even to the most rostral neuraxis.
Nominal cortical efferents cause and modulate both motor and autonomic responses for the benefit of systemic survival giving it insight, texture, and content. The vast majority of this outflow terminates in the areas of the cord that influence functions of the nervous system not under voluntary control, e.g. the regulation of heartbeat or gland secretions, while a relatively smaller percent of these same cortical efferents have their effect on the anterior horn of the cord ultimately exerting their influence on muscle function.
Regardless of their cortical tissue of origin, all the impact from the rostral neuraxis and segmental stimuli finally has its effect on the anterior horn of the cord, the final common pathway. This is where the viscerosomatic system parts ways to affect the muscles and organs according to that specific spinal cord segment, ultimately to cause their own afferent stimuli that finally terminates on the segmental dorsal horn of the cord.
Normalizing Efferent Autonomic Drive
The treatment goal in this procedure was to normalize the dysfunctional efferent autonomic drive secondary to deafferentation of the extrapyramidal and pyramidal outflow that arose as a consequence of a compromised primary afferentation.
CONCLUSION
There appears to be uniformity between functional relationships found with the standard CRS’s and the results displayed during normal manual muscle testing. The demonstrable pathological signs are consistent with a cortical deafferentation and a resultant erroneous motor response. A disruption is demonstrated as any functional display inconsistent with that which was anticipated as normal. These findings strongly indicate that the definition of CRSs should be expanded and used during a standard neurological examination, especially when their clinical significance is sustained and prominent in the examination.
Considering the foregoing, it behoves us to reconsider what it means to have a retained or re-appearing primitive reflex, i.e., reconsider that CRSs are invariably signs of significant brain injury, or at the least a deafferentation of their primary afferent(s). New thought suggests that CRSs are demonstrable in the functional nervous system and any modification of their display indicates a deafferentation of one of the potential neurological levels of dysfunction. The data in this case series suggested that re-establishing functional central and peripheral reciprocity facilitates cortical integrity. The universal goal is to re-establish issues of functional cortical reciprocity in order to achieve purposeful neurological display. Actually, there is much non-drug treatment that can be done for patients with dysfunctional CRS’s.
References
-
Bennett HP, Corbett AJ, Gaden S, Grayson DA, Kril JJ, Broe GA. Subcortical vascular disease and functional decline: A 6-year predictor study. J Am Geriatr Soc. 2002 Dec;50(12):1969-77.
-
Cuesta MJ, Peralta V, Zarzuela A, Calvo R, Garcia M, Serrano F. Neurological soft-signs in psychosis: threshold criteria for discriminating normal controls and for predicting cognitive impairment. Schizophr Res. 2002 Dec 1;58(2-3):263-71.
-
Egan MF, Hyde TM, Bonomo JB, Mattay VS, Bigelow LB, Goldberg TE, Weinberger DR. Relative risk of neurological signs in siblings of patients with schizophrenia. Am J Psychiatry. 2001 Nov;158(11):1827-34.
-
Gladstone DJ, Black SE. The Neurological Examination in Aging, Dementia and Cerebrovascular Disease. HealthPlexus, September 1, 2002.
-
Godde B, Ehrhardt J, Braun C. Behavioral significance of input-dependent plasticity of human somatosensory cortex. Neuroreport. 2003 Mar 24;14(4):543-6.
-
Gourion D, Goldberger C, Olie JP, Lôo H, Krebs MO. Neurological and morphological anomalies and the genetic liability to schizophrenia: a composite phenotype. Schizophr Res. 2004 Mar 1;67(1):23-31.
-
Graziano A, Jones EG. Early withdrawal of axons from higher centers in response to peripheral somatosensory denervation. J Neurosci, March 25, 2009, 29(12):3738-3748.
-
Guzik P, Bankes L, Brown TM. Acamprosate and Primitive Reflexes. Ann Pharmacother. 2007 Apr;41(4):715-8..
-
Hyde TM, Goldberg TE, Efan MF, Lener MC, Weinberger DR. Frontal release signs and cognition in people with schizophrenia, their siblings and healthy controls. Brit J Psychia. 2007; 191:120-5
-
Lawrie SM, Byrne M, Miller P, Hodges A, Clafferty RA, Cunningham Owens DG, Johnstone EC. Neurodevelopmental indices and the development of psychotic symptoms in subjects at high risk of schizophrenia. Brit J Psych (2001) 178: 524-530 Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999 Dec 21; 96(26): 15222-7.
-
Schott JM, Rossor MN, The grasp and other primitive reflexes. J Neurol Neurosurg Psychiatry 2003;74:558-560 doi:10.1136/jnnp.74.5.558
-
Schott JM, Rossor MN. The grasp and other primitive reflexes. J Neurol Neurosurg Psychiatry 2003;74:558-560 doi:10.1136/jnnp.74.5.558 Walterfang M, Velakoulis D. Cortical release signs in psychiatry. Aust N Z J Psychiatry. 2005 May;39(5):317-27.
-
Weiss T, Miltner WHR, Lieper, J, Meissner W, Taub E. Rapid functional plasticity in the primary somatomotor cortex and perceptual changes after nerve block. Eur J Neurosci. 2004 Dec;20(12):3413-23.

Cortical or frontal release signs are common in the general population, occurring in roughly a quarter of young healthy adults, and are more common with advancing age. (Gladstone & Black, 2002) Many researchers have correlated the relationship between primitive reflex display and cognitive impairment. Although a single CRS is of limited clinical significance, multiple CRSs tend to correlate with brain pathology. (Schott & Rossor, 2003) However, the commonly held notion that these reflexes should somehow disappear at certain times has a high probability of being inaccurate. (Walterfang et al, 2005)These reflex patterns are initially distinct and separate at various stages of human development and become integrated into the human neurological fabric consistent with its maturation. The mature neocortex normally keeps higher functional neurological patterns intact. The indications of cortical escape are the motor display of a cortical dysfunction and as such are considered abnormal. Their accumulative effect as a result of a more rostral dysfunction eventually causes a freeing of a group of cortical patterns that later display themselves in the context of central nervous system disease. The clinical display of these CRSs signifies severe neurological damage when found in the adult, but those same findings may also suggest that they are related to a deafferentation syndrome and can be exposed with manual muscle testing. The interpretation of each CRS is described herein with reference to functional neurological norms.The clinical significance of the demonstrable GR alone, for example, [or in combination with the asymmetric tonic neck reflex, Babinski-like (flexor withdrawal response), and crossed extensor reflexes], as well as their contribution to the early and differential diagnoses of several functional dysfunction syndromes, have been demonstrated in the clinical environment. Moreover, patients with five or more abnormal physiological reflexes have a greater probability of